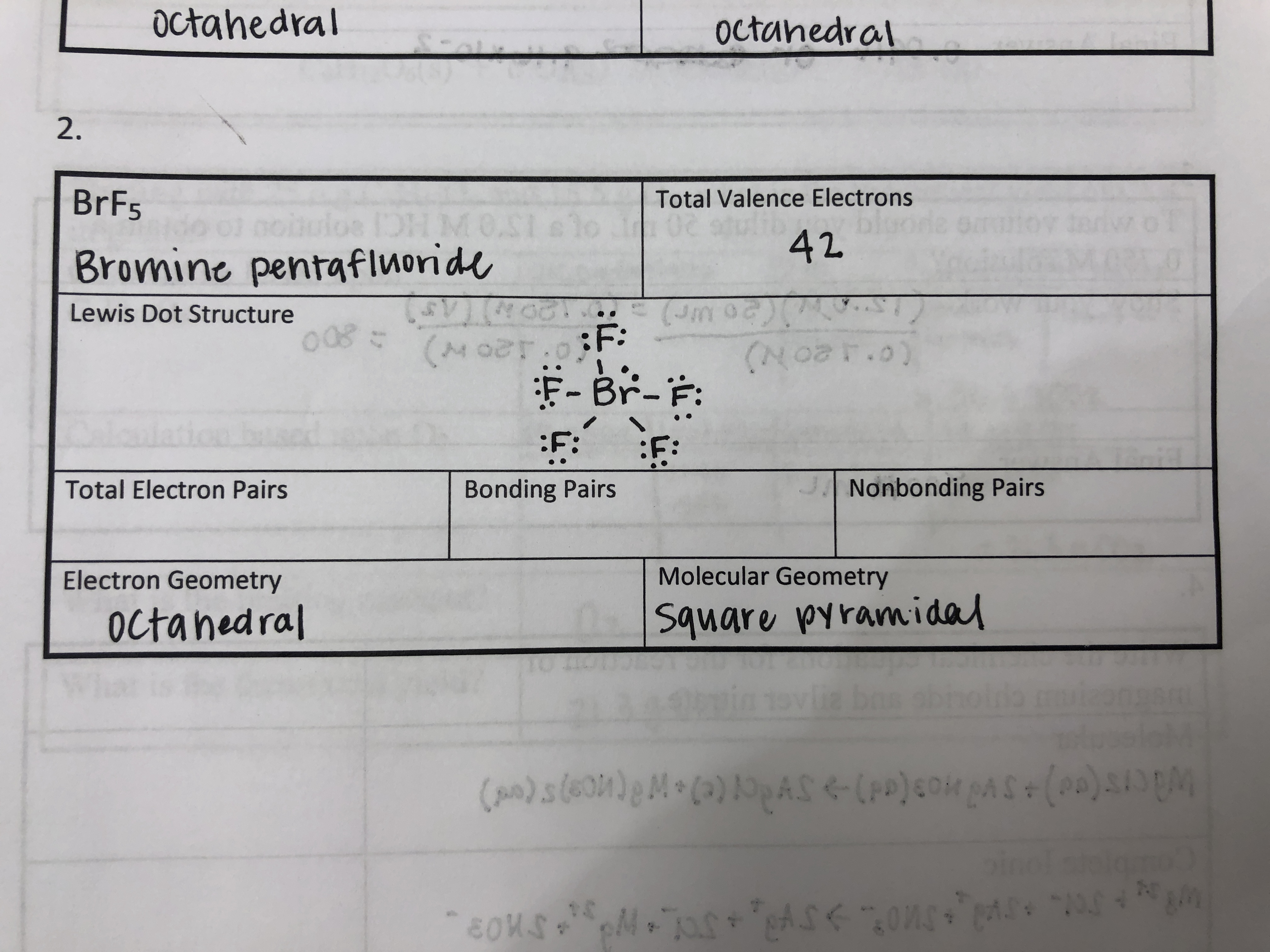
Bromine pentafluoride can be formed either by (1) the direct reaction of bromine with excess fluorine above 150 °C or by (2) the reaction of BrF3 vapor and gaseous fluorine at 200 °C. Frim R, Ukeles SD; Bromine, Inorganic Compounds. Bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule. The compound cyanogen (CN)2 has covalent bonds shown in this diagram. How many electrons are shared between one nitrogen atom and one carbon atom? Valence Electrons Questions What are valence electrons? The Valence Electrons are a type of outer shell electron. Which can participate in the formation of a chemical bond and attach to an atom even if the outer shell is not closed. A step-by-step explanation of how to draw the CaBr2 Lewis Dot Structure.For CaBr2 we have an ionic compound and we need to take that into account when we dra.
Bromine Valence Electrons
Bromine Pentafluoride Valence Electrons

Click to see full answer
Hereof, does bromine lose or gain electrons?
Bromine atoms tend to gain just one electron to get to a full octet, as Bromine is in Group VII. A chemical consisting of an aluminum ion and a bromide ion in their stable states would be AlBr2+, but it is not an ionic compound because it has a charge. Thus it tends to lose two electrons.
Also, how many electrons are gained or lost in iodine? Moving from neutral to +3 charge means it just lost 3 electrons. (Electrons have negative charge.) Similarily, 6 iodine atoms go from -1 to 0, thus gaining one electron.
Also to know is, what is the number of electrons gained or lost in chlorine?
It is now referred to as a sodium ion. Chlorine (Cl) in its lowest energy state (called the ground state) has seven electrons in its outer shell. Again, it is more energy-efficient for chlorine to gain one electron than to lose seven.
What is an atom that gains or loses one or more electrons?

Valence Electron Br
An atom that gains one or more electrons will have a NEGATIVE charge. An atom that loses one or more electrons will have a POSTIVE charge. An atom that gains or loses one or more electrons is called an ION. A positive ion is called a CATION and a negative ion is called an ANION.
Click to see full answer
Bromine Valence Electrons Need
Similarly, what happens when a bromine ion becomes an ion?
IONS Bromine Can MakeTo become an ion, an element has to gain or loose electrons. If it gains electrons, it receives a negative charge because it then has more electrons than protons. This is known as an anion. If it looses electrons, it receives a positive charge because it has more protons than electrons.
Furthermore, does bromine gain or lose electrons? Bromine atoms tend to gain just one electron to get to a full octet, as Bromine is in Group VII. A chemical consisting of an aluminum ion and a bromide ion in their stable states would be AlBr2+, but it is not an ionic compound because it has a charge. Thus it tends to lose two electrons.
Also question is, will a bromine atom form a positive or negative ion Why?
The neutral atom of bromine has 35 electrons because the number of electrons equals the number of protons. c) Bromine gains an electron, what is the resulting ion called and is it positively or negatively charged? When bromine gains an electron, the resulting ion is called an anion and is negatively charged.
Bromine Valence Electrons
What type of ion does bromine form?
Bromine Valence Electron Configuration
A bromide is a chemical compound containing a bromide ion or ligand. This is a bromine atom with an ionic charge of −1 (Br−); for example, in caesium bromide, caesium cations (Cs+) are electrically attracted to bromide anions (Br−) to form the electrically neutral ionic compound CsBr.