To see all my Chemistry videos, check outis atomic mass? It is a weighed average of the different isotopes of an element. The average mass of an element based on the percent abundance of each isotope of that element. (atoms with different number of neutrons, but same number of protons). What is the average atomic mass of Boron if it exists as 19.90% boron-10 and 80.10% boron-11?
What is the average atomic mass of silicon?
Average Atomic Mass 1 Average Atomic Mass How are the masses on the periodic table determined? Most elements have more than one naturally occurring isotope. As you learned previously, the atoms of those isotopes have the same atomic number (number of protons), making them belong to the same ele-ment, but they have different mass numbers (total number of protons and neutrons) giving. This is a list of chemical elements, sorted by atomic mass (or most stable isotope) and color coded according to type of element.Each element's atomic number, name, element symbol, and group and period numbers on the periodic table are given. The number in parenthesis gives the uncertainty in the 'concise notation' dis given in parenthesis next to the least significant digits to which it. To understand how different isotopes affect average atomic mass let's look at the example of carbon. Carbon atoms come in three different versions; carbon-12, carbon-13 and carbon-14. The isotope has its own mass and relative abundance (how common it is) Carbon-12 atoms have a mass of 12 atomic mass units (amu) and makes up 98.93% of all carbon atoms. Carbon-13 atoms have a mass of 13.003amu.
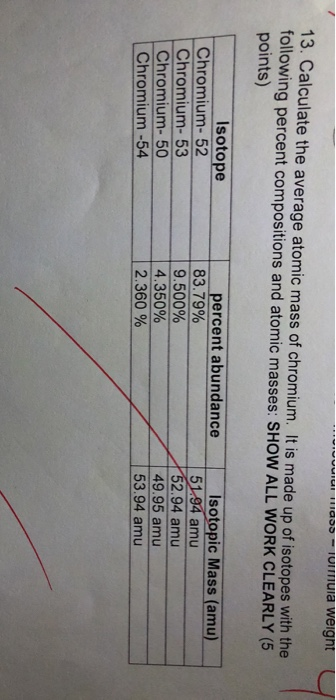
Given the following data, calculate the average atomic mass of silicon.
isotope = Si-28
amu =27.9769
abundance(%) =92.18
isotope = Si-29
amu =28.9765
abundance(%) =4.71
isotope =Si-30
amu =29.9738
abundance(%) =3.12
Given the following data, calculate the average atomic mass of silicon.
isotope = Si-28
amu =27.9769
abundance(%) =92.18
isotope = Si-29
amu =28.9765
abundance(%) =4.71
isotope =Si-30
amu =29.9738
abundance(%) =3.12
1 Answer
Multiply the amu by the percentage of occurrence to arrive at an average atomic mass of
Explanation:

We take the amu of each isotope, multiply it by the percentage of occurrence, and end up with a weighted average:

[{Blank}] Is The Average Mass Numbers Of All Isotopic ...
This simplifies to:
Checking 'the internet' to verify our answer, I found 28.0855 u ± 0.0003 u, so the answer calculated for our question is pretty close.
Cached
Related questions
